There are two types of drugs on the market: orthosteric drugs and allosteric drugs. The major difference between the two is where they bind on the protein or enzyme to enable a chemical reaction for the drug to provide its therapeutic effects.
Allosteric drugs are advantageous because their unique binding mechanism prevents unexpected harmful side effects and overdosage. However, their potential for drug discovery is impeded by the lack of molecular understanding of how they operate.
New findings, in press in Nature Communications, offer game-changing insight.
In the study, researchers at Yale Chemistry have uncovered a key condition of signaling and communication that activates allostery on effector binding.
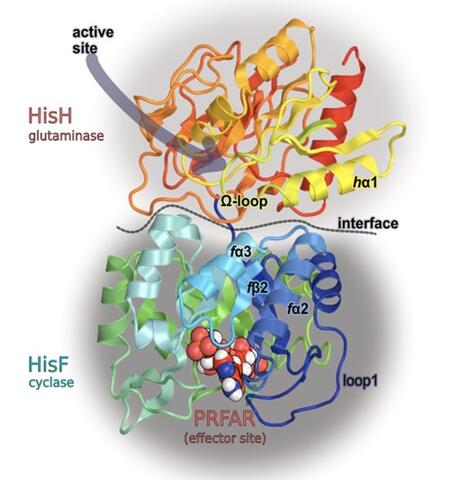
“Allostery is a mechanism in enzymes and proteins, where a chemical compound, typically a small molecule, binds at one site that’s far away from the active site that does chemistry, but the small molecule can influence that distant site,” said Patrick Loria, professor of chemistry and of molecular biophysics and biochemistry. “Somehow, those two sites, even though they are far apart, communicate with each other.”
Unlike orthosteric drugs, which bind to the active site, allosteric drugs selectively bind to those sites far from the active site, providing certain advantages against off-target effects and overdosage. One can see why these drugs are desirable.
In the study, the researchers used molecular dynamics simulation and nuclear magnetic resonance (NMR) spectroscopy to investigate how temperature increases affect allostery in the enzyme imidazole glycerol phosphate synthase (IGPS). They demonstrated that a temperature increase from 30° C to 50° C in the apo state of IGPS could activate a structural and dynamical pattern that remarkably resembles the effector-induced allosteric activation. Furthermore, they also identified residues along the signaling pathway and their role in temperature-induced allostery.
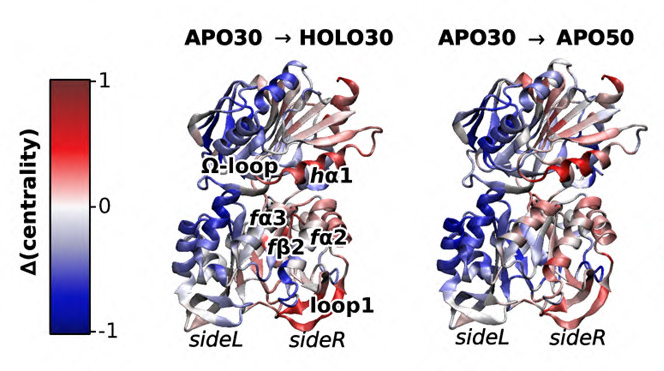
“The temperature dependence of the activated enzyme and the unactivated enzyme were different, and we didn’t really expect that,” said Loria. “What that means is the allosteric activator isn’t as good as it could be at the growth temperature of that organism.
Upon investigating that, we made a novel observation that increasing the temperature does the same thing to allostery as the normal small molecule does. So, temperature is kind of like a surrogate for the small molecule activator.”
The big picture is that these findings provide a better understanding of how allostery works, which is important in discovering a new avenue for controlling catalytic activity and enzymes by modulating the allosteric pathway.
Overall, this study opens doors to developing novel tools to control IGPS activity, such as rationally designed allosteric drugs, antipathogens, and engineered variants.
This research was a collaboration between the labs of Professor Patrick Loria and Victor Batista, John Gamble Kirkwood Professor of Chemistry. Apala Chaudhuri, a graduate student from the Loria Lab, did the NMR experiments, and Federica Maschietto, a postdoctoral scientist from the Batista Lab, did much of the computational work.
“It’s amazing that we can figure out how an enzyme from a thermophilic bacterium behaves when it is heated up,” said Batista. “It’s like the enzyme is being activated by a chemical!”
For the past decade, Loria and Batista have worked together to understand how allosteric sites communicate and to identify the network of residues and the pathways between the two sites. That work laid the foundation for their current investigations on allosteric mechanisms.
Contributing authors Federica Maschietto, Apala Chaudhuri, J. Patrick Loria, and Victor S. Batista are from Yale. Other contributing scientists are from the International Center for Theoretical Physics, the National Institutes of Health’s Laboratory of Computational Biology, National Heart, Lung and Blood Institute, the Université de Lyon, and the Universita di Bologna.
Learn more about biophysical chemistry (Loria Lab) and physical chemistry (Batista Lab).